Research & Breakthroughs (R&B)

Innovation Project-1: Advanced Cardiovascular Research
(In progress)
Introduction
Cardiovascular diseases are one of the main reasons behind deaths according to recent reports. Approximately, 92.1 million American adults have at least one type of cardiovascular disease. 30% of adults with an existing cardiovascular condition suffer from heart disease. For the last century, cardiovascular diseases have been considered the main cause of death in the United States. Treatment or countermeasure options may vary depending on the severity and type of the condition. Altering lifestyle habits, such as weight control, increasing physical activity, quitting the consumption of tobacco products, moderating alcohol intake, and decreasing saturated fat and sodium in the diet, are beneficial to improving cardiovascular health. Moreover, assistive medication for high blood pressure or cholesterol is also beneficial. For people who have been diagnosed with severe conditions, lifestyle changes, and assistive medication may not be enough. Heart transplantation could be considered the best possible treatment. Yet, the ratio of available heart donors to people on the transplantation waitlist is not promising. The World Health Organization’s “Atlas of Heart Disease and Stroke” foresees a grim future for the World’s population. One of the predictions of the report is that by 2030 globally 32.5 % of all deaths would be caused by cardiovascular diseases. The report also plants hope by stating that cardiovascular diseases could be prevented considering research conducted for the last 50 years. Therefore, there is an imminent need for the development of new treatments for cardiovascular diseases and the development and testing of proposed future treatments. The development of a novel Cardiovascular Performance Benchmark Platform (CPBP) would be a perfect candidate to meet the requirements of such a need.
Goal
The hypothesis will be tested using the following specific aims as shown in Figure 1: (01) 3D printing customized specialized biocompatible, silicone-based heart valve manufacturing. (02) Silicone heart valve testing using the MCL system for functionality and performance. (03) Integrating a validated heart valve with the cardiovascular performance simulation platform to create a design and monitoring tool can lead to better and more physiologically
accurate research and development efforts.
02. Mock Circulation Loops (MCL) to Test Heart Valve Functionality
As shown in Figure 3, mock circulation loops are closed-loop piping systems whose sole purpose is to regenerate physiological circulatory parameters. MCLs consist of compliance chambers, resistance valves, artificial heart valves, and more importantly, a pump to act as actuators mimicking the ventricles.
Figure 3 Prototyped MCL system (adapted from Baturalp and Ertas, 2015).
03 Cardiovascular Performance Simulation Platform (CPSP)
CPSP will consist of a multistage computational model and an experimental setup for not only validation of the computational counterpart, but also serving as a test bed for cardiovascular assistance devices. The general stages of the computational part of CPSP could be listed as follows (see Figure 4): finite element analysis to identify mechanical deformation characteristics of the left ventricular simulator, fluid-structure interaction model to predict cardiac output and flow through valves, and computational fluid dynamics model to monitor flow from the aorta to the left atrium, excluding pulmonary flow.
(01) Design and Development of a 3D Printing Machine to Manufacture Customized Silicone Heart Valve
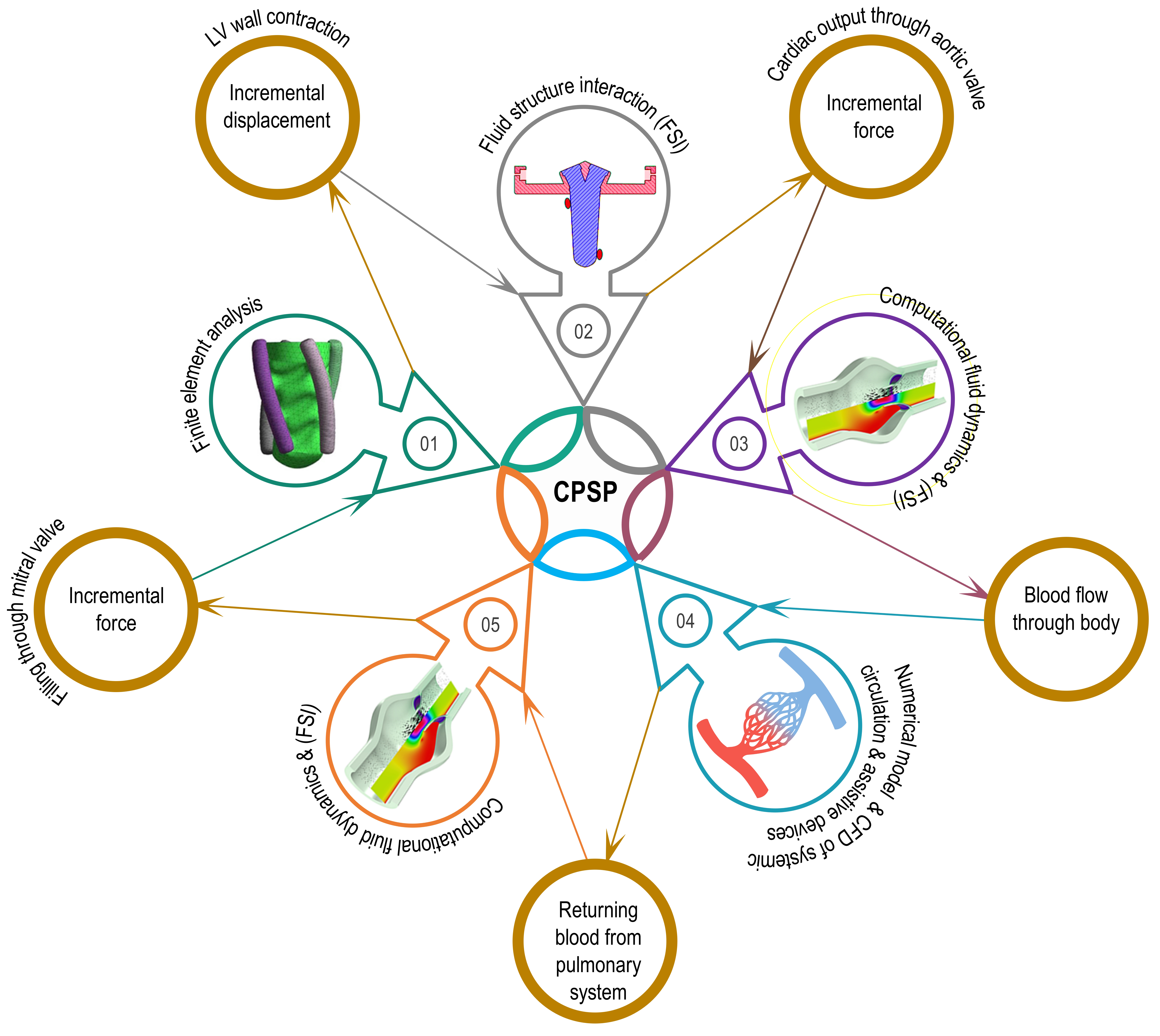
Figure 4 Proposed Cardiovascular Performance Simulation Platform. (adapted from Gulbulak and Ertas, 2019).

Figure 2 3D silicone printing machine final product (Ertas et al., 2023).
3D Printing of Silicone Heart Valve: A Major Breakthrough
04. Development of Left Ventricular Assistance Devices (LVAD)
The design of cardiovascular assistance devices, specifically left ventricular assist devices (LVADs), has not achieved perfection and has yet to fulfill its initial promises. The complex and sensitive nature of the working conditions of cardiovascular assistance devices is the main reason for their decelerated progress. For most individuals around the globe, future treatments seem to be the sole viable option for destination therapy.
Concluding Remarks
Related Published Articles
Ertas, A., Erik Farley-Talamantes, H. Saker, A. Rajan, C. Lamb, D. Flores, B. Norton. Innovative Approach to Design and Development of a 3D Silicone Printing Machine Using Transdisciplinary Integrated Design Tools. Transdisciplinary Journal of Engineering & Science, Vol. 14, pp. 19-63, 2023.
Gulbulak, U., Gecgel, O., and Ertas, A. A Deep Learning Framework to Approximate the Geometric Orifice and Coaptation Area of Polymeric Heart Valves Under Time-Varying Transvalvular Pressure. Journal of the Mechanical Behavior of Biomedical Materials, 2021, 117:104371. doi: 10.1016/j.jmbbm.2021.104371.
Gulbulak, U., Ertas, A., Pavelka, T., Baturalp, T. The Effect of Fundamental Curves on Geometric Orifice Area of Polymeric Bioprosthetic Heart Valves. Journal of the Mechanical Behavior of Biomedical Materials, 112, 104039. https://doi.org/10.1016/j.jmbbm.2020.104039.
Gulbulak, U., Ertas, A. Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator. Bioengineering, 2019, 6, 83; doi:10.3390/bioengineering6030083
Pavelka, T., Gulbulak, U., Baturalp, T., Ertas, A. Performance Evaluation of a Mock Circulation Loop with Bioprosthetic Heart Valves and a Beating Left Ventricle Simulator. Rice University, Houston, Texas, "Gold Coast Undergraduate Research Symposium," National, peer-reviewed/refereed. (2019).
Baturalp, T. B., Ertas, A. State of the Art Mock Circulation Loop and a Proposed Novel Design (pp. 23-29, 2015). Proceedings of the International Conference on Biomedical Engineering and Science (BIOENG'15).
Ertas, A., Farley-Talamantes, E., Cuvalci, O., and Gecgel, O. 3D-Printed Artificial Aortic Heart Valve Using UV-Cured Silicone: Design and Performance Analysis. Bioengineering 2025, 12, 94. https://doi.org/10.3390/bioengineering12010094
Innovation Project-2: A Novel Concept of Intermittent Peanut Drying Technology: Industrial Innovation to Enhance Biodiesel Production
An Overview of Innovation
As a "zero waste" industry, the peanut sector maximizes the use of every component of the plant, from the roots to the shells, thereby ensuring that no part goes to waste. Peanuts, recognized for having the lowest carbon footprint and minimal water needs among nuts, present an advantageous option for agricultural producers. Additionally, the peanut plant possesses a unique ability to enhance soil quality, benefiting the growth of other crops (National Peanut Board, June 2, 2023). Beyond their sustainability, peanuts serve as a valuable addition to health-conscious diets, offering high-protein nutrition while promoting environmental responsibility.
The environmental impact and pollutant control associated with renewable energy technologies remain pressing issues. Peanut can be utilized as a source for producing clean, renewable energy in various capacities. Peanuts, in addition to numerous other renewable energy sources, serve as a viable renewable energy option for the transportation sector, offering a sustainable alternative for powering vehicles. Biodiesel produced from peanut oil acts as a substitute for traditional fossil fuels in various modes of transportation, including cars, trucks, buses, railways, and agricultural machinery that have typically relied on petroleum-based diesel. With peanuts containing approximately 40-60% oil by weight, they are an outstanding source of biodiesel.
The costs and environmental impacts linked to fuels derived from petroleum, such as diesel, have generated considerable interest in developing sustainable biodiesel production systems within agricultural settings. A study by Butts et al. (2008) with soybean oil found that Research conducted in the field has shown that peanuts can yield 1138 kg/ha of peanut oil for $0.35 per liter. Should the price of off-road diesel be $0.92 per liter, it would be feasible to allocate 0.57 ($0.92-$0.35 = $0.57) per liter towards the activities of peanut combining, curing, storing, shelling, and crushing the peanuts, followed by the conversion of the oil into a methyl ester (biodiesel). The average costs incurred from the processes of combining ($124 per hectare), curing ($74 per ton), and shelling ($148 per ton) of farmer-stock peanuts result in an estimated processing expense of $0.66 per liter of extractable peanut oil (Butts et al., 2008) This figure exceeds the budgeted amount of $0.57 per liter for peanut oil. Reducing the costs associated with peanut processing necessitates the development of a cutting-edge peanut drying technology that can generate biodiesel from peanuts on a large scale in a more efficient timeframe, thereby supporting biodiesel production. The system should be designed to minimize the handling of peanuts to the greatest extent possible while simultaneously improving the quality of the final cured product.
The essential technologies and opportunities for substantially lowering peanut processing costs have not been fully realized. Numerous companies utilize various adaptations of Ertas’ 1999 semi-trailer peanut drying system. Considering their significance as outlined in the previous paragraph, this research examines a new strategy for the peanut curing process, specifically an inventive design for an intermittent peanut drying system intended to boost energy efficiency, elevate quality, and decrease the expenses involved in peanut curing.
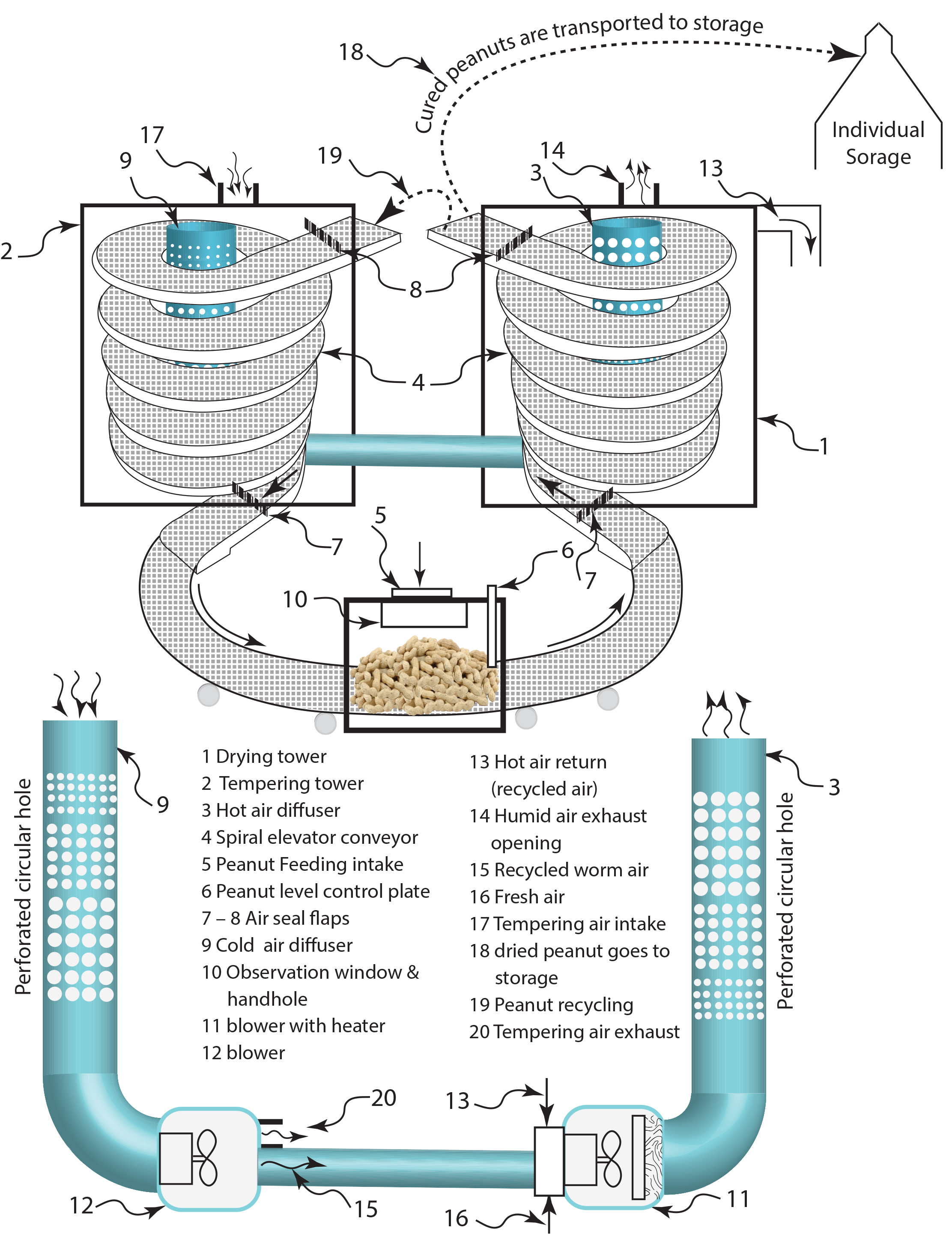
A novel intermittent peanut curing system featuring a spiral conveyor, shown in Figure 1, is introduced. This proposed system ensures low maintenance, efficient, and reliable operation by utilizing spiral elevator conveyors (4) for the processes of drying and tempering, all while occupying minimal workspace.
This proposed invention consists of two thermal treatment towers equipped with reverse-rotating spiral conveyors: a drying tower (1) and a tempering tower (2). Peanuts are introduced through the peanut feeding intake (5) at a specific height, regulated by the level control plate (6). The peanuts are then conveyed into the drying tower (1) via a spiral conveyor (4). As the peanuts ascend through the spiral conveyor, heated air extracts moisture, which is expelled through the humid air exhaust openings (14). A portion of this heated air is redirected (13) back to the intake of the blower (11) enclosure. The drying air diffuser (3) ensures uniform air distribution, while linearly arranged perforated circular holes facilitate consistent drying airflow, thereby maintaining the quality of the peanuts.